Abstract
Introduction/ Background
Traditionally, MM harbouring t(11;14) is considered standard risk disease. In the era of novel therapies, however, pts with t(11;14) are reported to have inferior outcomes to other standard risk pts. Evidence of response to the BCL-2 inhibitor, Venetoclax (Ven), has further increased interest in understanding outcomes of t(11;14) pts. In this study, we aimed to describe the historical outcomes of t(11;14) MM and response to Ven in a real-world cohort of Australian pts.
Methods
This was a retrospective, multicentre study conducted by members of the Australasian Leukaemia and Lymphoma Group, Myeloma Working Party. Cases were identified by interrogation of cytogenetics/FISH database from 2010 to 2019 inclusive. Baseline patient and disease characteristics, treatment exposure and outcomes were extracted from hospital medical records. Descriptive statistics, and survival analyses were performed as appropriate.
Results
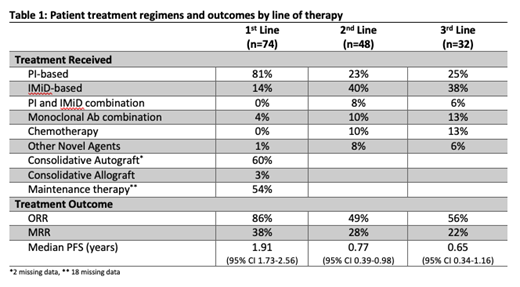
Seventy-four pts [median age 65 years (yrs), range (43-85)] were identified across seven centres. 43% pts had ISS Stage III MM with 88% harbouring additional cytogenetic abnormalities, incl. 13% with gain in 1q and 12% with del(17p). The majority (81%) of pts received proteasome inhibitor (PI)-based 1 st line therapy with 60% having an upfront autologous stem cell transplant (ASCT) and 54% having immunomodulatory drug (IMiD)-based maintenance therapy. Two patients received an allogeneic stem cell transplant after ASCT. The overall response rate (ORR) was 86% with 38% achieving very good partial response (VGPR) or better. The median progression free survival-1 (PFS-1) was 1.91 yrs (95% CI 1.73-2.56) [PI-based, n=60, PFS 1.84yrs (95% CI 1.61-2.41) vs IMiD-based, n=5, PFS 4.58yrs (95% CI 1.16-5.51), HR 0.68 p=0.45] The median overall survival (OS) was 5.35 yrs (95% CI 4.12-6.56). Second and third line therapy was predominantly IMiD-based (see Table 1) with recent introduction of anti-CD38 monoclonal antibodies (mAbs). Median PFS-2 was 0.77 yrs (95% CI 0.39-0.98) while median PFS-3 was 0.65 yrs (95% CI 0.34-1.16). Eleven pts (median 3 prior lines of therapy) were given Ven [Six pts in combination with PI, three with PI and mAbs and two with dexamethasone]. ORR to Ven was 55% with 45% achieving VGPR or better. Median PFS with Ven was 0.54 yrs (85% CI 0.05-2.17). Median PFS for patients with 1-4 lines (n=6) was 1.22 years and median PFS for patients with 5 lines or more (n=5) was 0.54 years, HR 0.56 (95% CI 0.12-2.5).
Conclusion
For pts harbouring t(11;14), the duration of response to PI-based 1 st line therapy is suboptimal even with the use of ASCT consolidation in the majority of patients. Despite multiple prior lines of therapy, Venetoclax shows promising results and every effort should be made for early identification of this cytogenetic lesion. Further studies are required to examine the impact of novel triplet and quadruplet combination therapy and use of venetoclax early in the disease course of this sub-group of patients.
Talaulikar: Takeda: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Jansenn: Honoraria, Research Funding; Roche: Honoraria, Research Funding; EUSA Pharma: Honoraria, Research Funding. Gibbs: Janssen, Celgene, Amgen, Takeda, BMS and Pfizer: Consultancy, Honoraria; AbbVie: Consultancy.